Japan Unveils Breakthrough Manganese Catalyst for Green Hydrogen
Japan’s RIKEN Institute has delivered a major breakthrough in the search for affordable green hydrogen by developing a manganese-based catalyst that could entirely displace costly noble metals like platinum and iridium in PEM (proton exchange membrane) electrolysers. These electrolysers are crucial for splitting water into hydrogen in acidic conditions but have long relied on scarce precious metals, severely limiting scalability. RIKEN’s team, led by Ryuhei Nakamura, focused on manganese oxide (MnO₂), an abundant and inexpensive material, and discovered that tweaking its atomic lattice—specifically boosting the proportion of “planar” oxygen sites—dramatically enhances both activity and durability. In fact, the optimized catalyst sustained the oxygen evolution reaction at 200 mA/cm² for over 1,000 hours in a real PEM electrolyser, generating roughly ten times more hydrogen than earlier non-noble-metal catalysts.
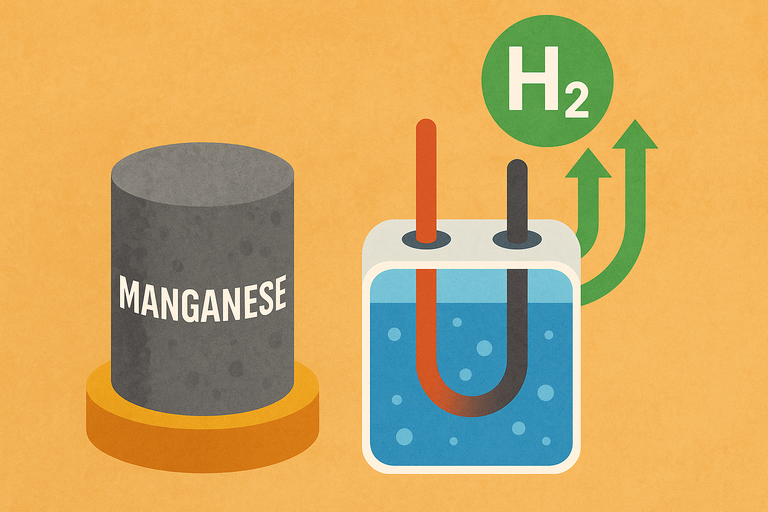
Even more impressively, at a current density of 1,000 mA/cm², it maintained performance for a month, transferring 100× more charge than previous manganese oxides.
This advance builds on prior work: nearly two years ago, the same team embedded manganese into a cobalt oxide structure to eliminate reliance on rare metals—though stability was insufficient for real-world PEM use.
Their latest iteration, by contrast, achieves longevity without compromising performance: as co-author Shuang Kong stated, “Surprisingly... the improved stability did not come at a cost in activity”.
The innovation has far-reaching implications. PEM electrolysers are seen as key to converting intermittent renewable energy into storable hydrogen for heavy industry, transport, and grid balancing—but their adoption has been bottlenecked by the high cost and supply scarcity of iridium. RIKEN highlights that scaling PEM systems to terawatt levels would require four decades’ worth of global iridium production—an unsustainable scenario.
By using manganese, available at millions of times the abundance of iridium, this work could revolutionize cost structures and supply chains, accelerating green hydrogen adoption.
The team isn’t stopping there. They’re actively exploring ways to push current densities beyond 200 mA/cm² and achieve catalyst lifetimes measured in years rather than months—all essential milestones for industrial implementation.
They’re also investigating hybrid catalysts: one project combines atomically dispersed iridium on manganese oxide—enough to slash iridium usage by 95% while maintaining performance, lasting 3,000 hours at 82% efficiency. Industrial collaboration is already underway to refine and scale these materials.
Ultimately, this manganese-centric breakthrough could transform green hydrogen from a costly niche to a mainstream energy solution. By removing the precious-metal barrier, the path opens for PEM electrolysers to be deployed at scale—powering zero-emission vehicles, decarbonizing heavy industry, and enabling reliable storage of renewable energy. It’s a pivotal advance in our journey toward a carbon-free energy economy—and proof that, sometimes, the most powerful breakthroughs begin with big ideas in the tiniest atomic rearrangements.
How might this breakthrough in catalyst design reshape global supply chains and the geopolitics of clean energy?
https://www.reddit.com/r/goodnews/comments/1lgwj6q/japan_unveils_breakthrough_manganese_catalyst_for/
This post has been shared on Reddit by @tsnaks through the HivePosh initiative.